Research
The development of π-conjugated compounds is at the forefront of research to benefit from their unique optical and semiconducting properties and pay the way for the emergence of organic electronic applications. The unique advantage of organic materials vs inorganic counterparts is their structural tunability with respect to the desired applications. Extension of π-delocalization from small molecules to one-dimensional (1D) polymers produce superior optical and electronic properties. However, further extension of π-delocalization to the second dimension (2D) is expected to give rise to even more fascinating properties.
Our research group focus on discovering novel π-conjugated molecules (small molecules, Oligomers, and one-/two-dimensional polymers) having special electronic and optical properties. Further, we explore the potentiality of the developed systems for the application in sensing (fluorescent, electronic and chemiresistive), conductivity, photocatalysis and electrochromism etc.
- 𝝅-conjugated organic and inorganic compounds for optoelectronics
- Polycyclic aromatic hydrocarbons with ground state open shell biradicals
- NIR-absorbing and emissive materials
- Organic fluorescent materials for ion and explosives sensing
- 𝝅-conjugated two-dimensional organic polymers
Selected Abstracts
π-conjugated small molecules
Synthesis of p-Azaquinodimethane-based Quinoidal Fluorophores
DOI: 10.1021/acs.joc.3c01342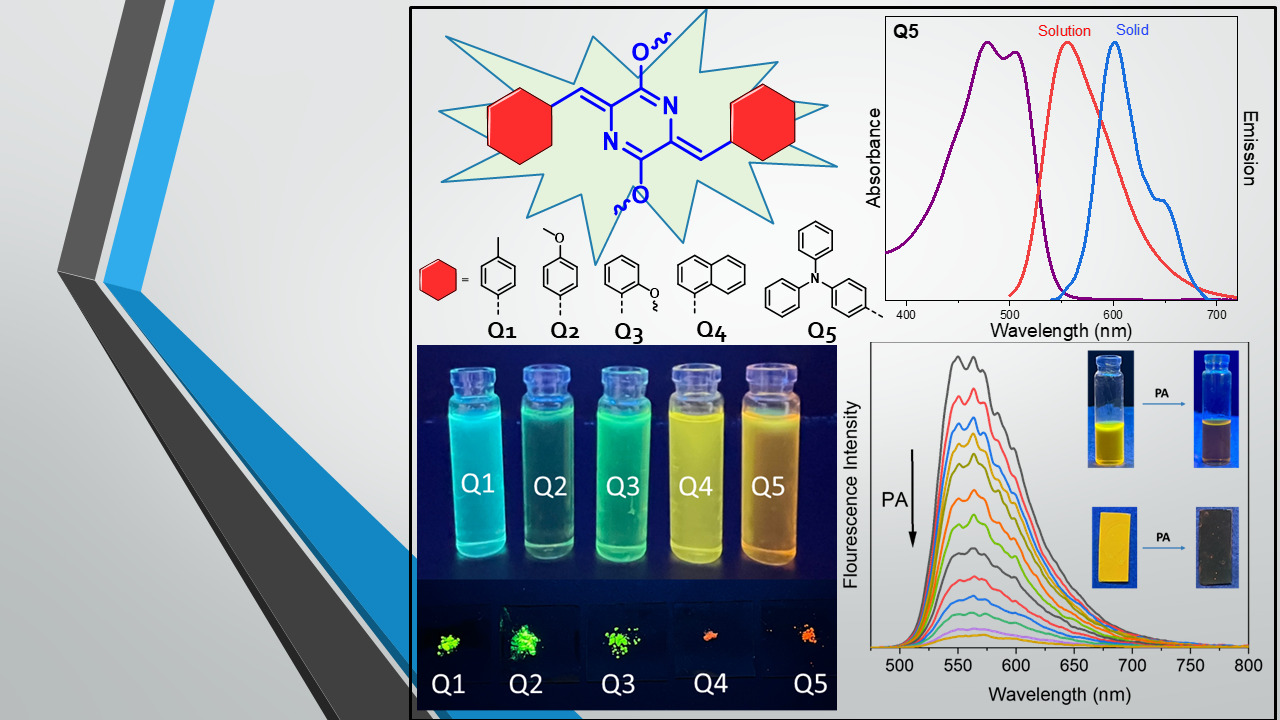
Pd-Catalysed Direct Arylation of Distyrylbenzene: Strong Dual-state Fluorescence and Electrochromism
DOI: 10.1002/202400015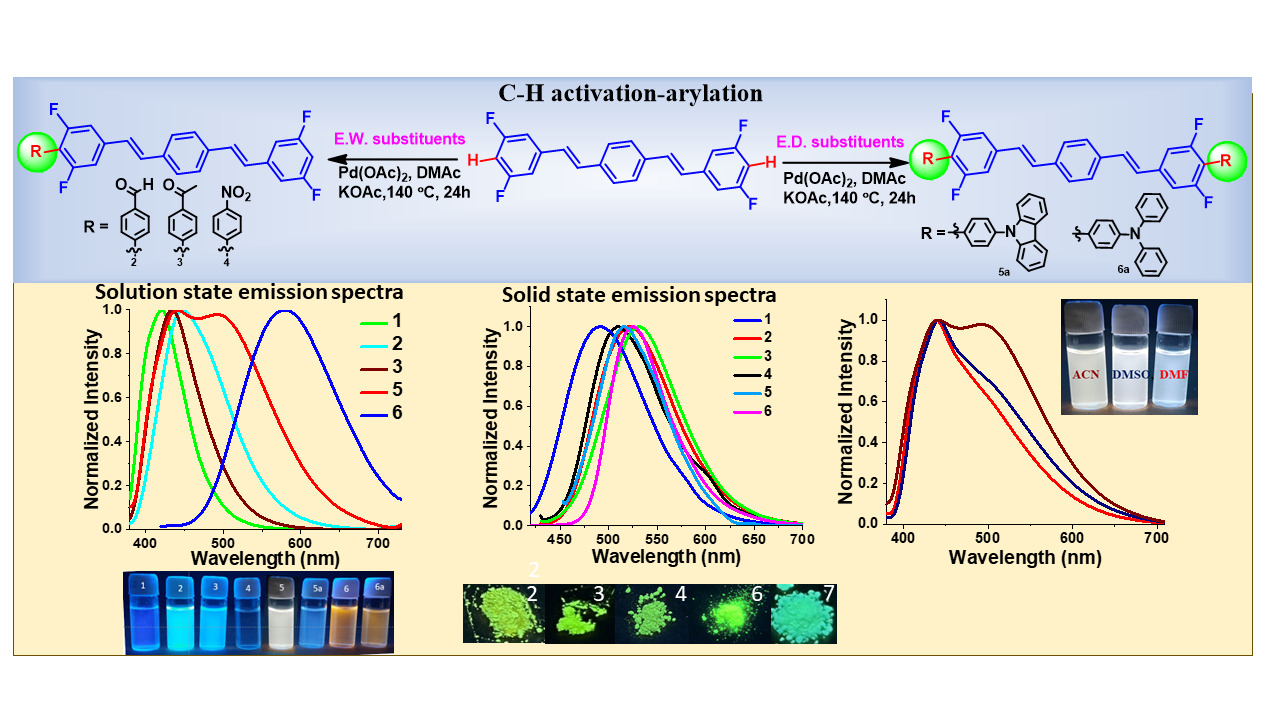
Synthesis of tetrazine-tetracyanobutadienes and their transformation into pyridazines via inverse-electron demand Diels–Alder cycloaddition (IEDDA)
DOI:10.1039/D3OB00595J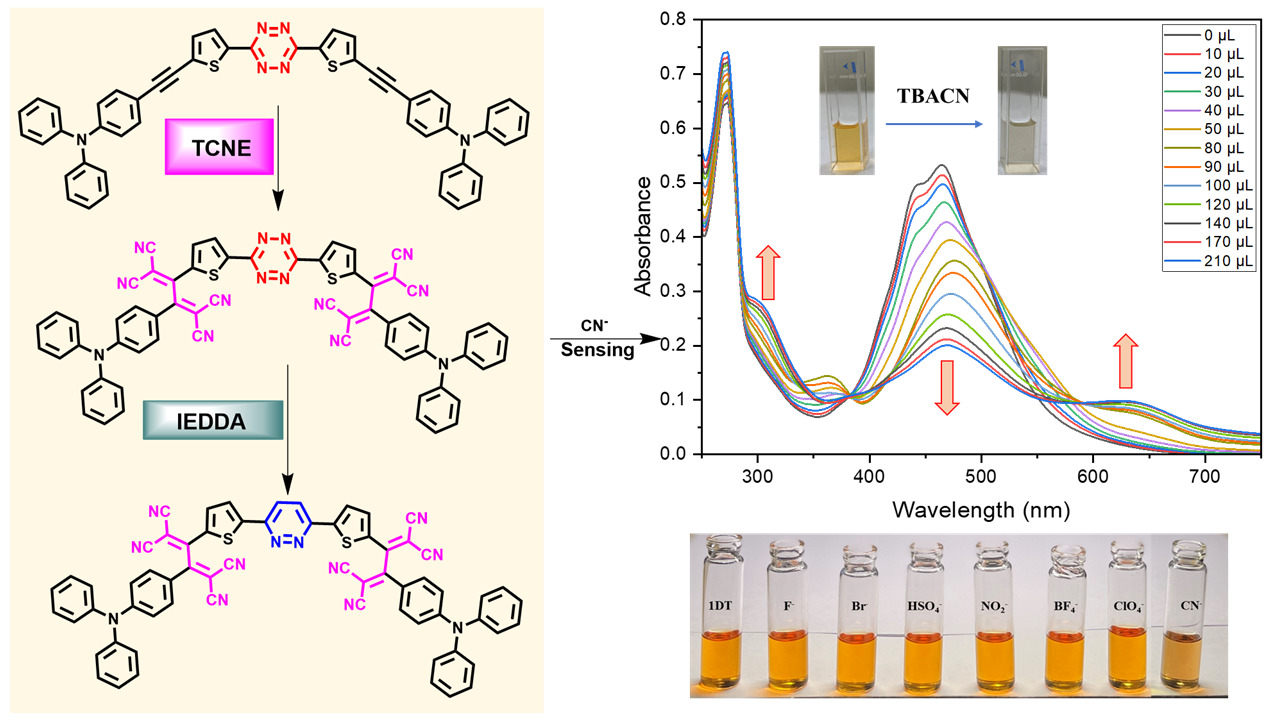
Vinylene and cyanovinylene thiophene compounds via C-H activation
DOI:10.1039/D30B00560G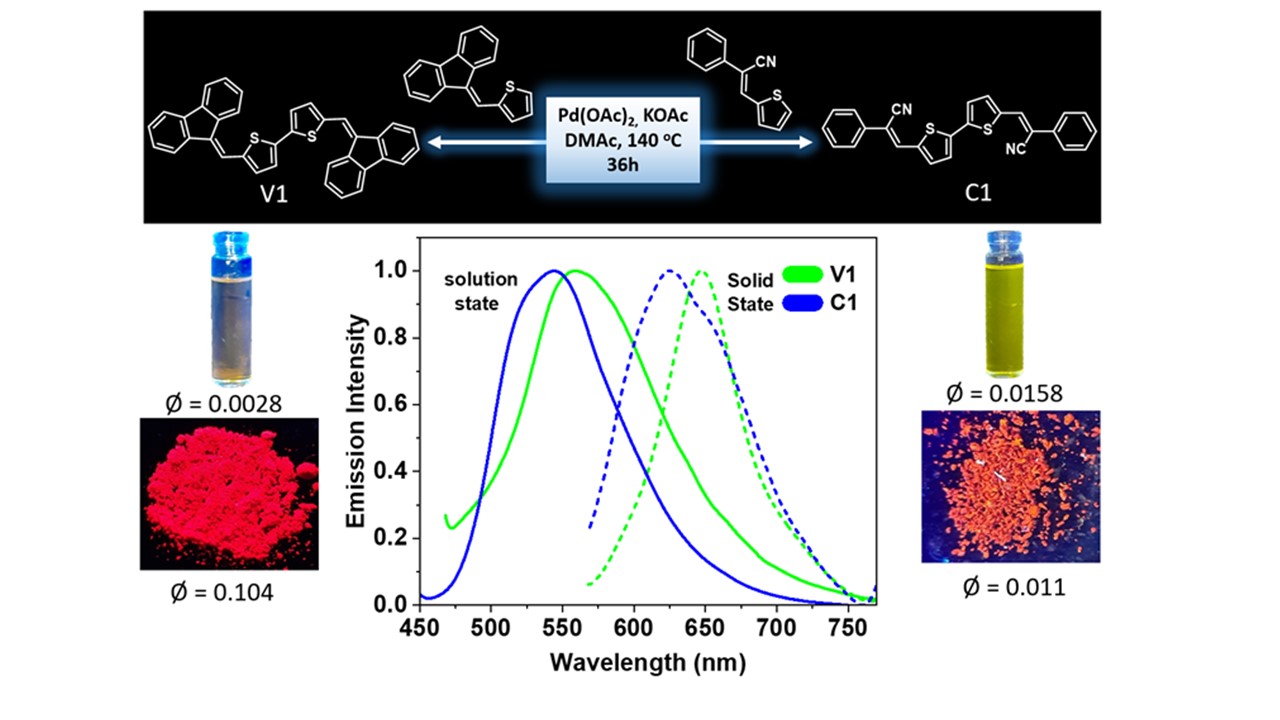
Vinylene-linked diketopyrrolopyrrole chromophores for electrochromism
DOI: 10.1039/D4RA01280A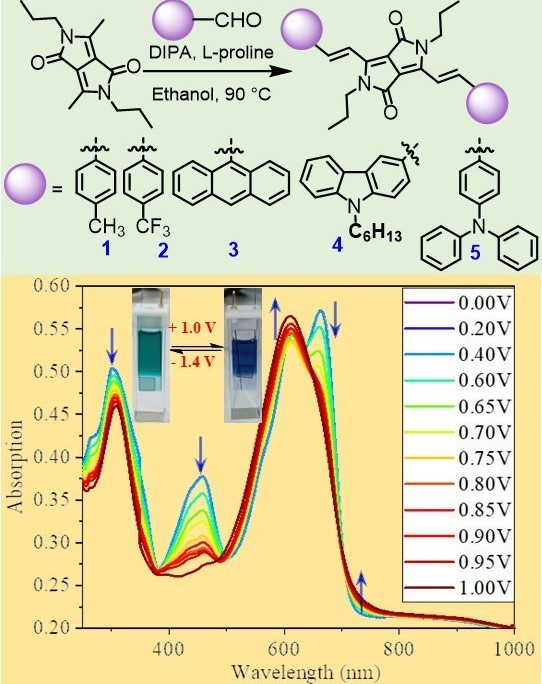
1D π-conjugated molecules
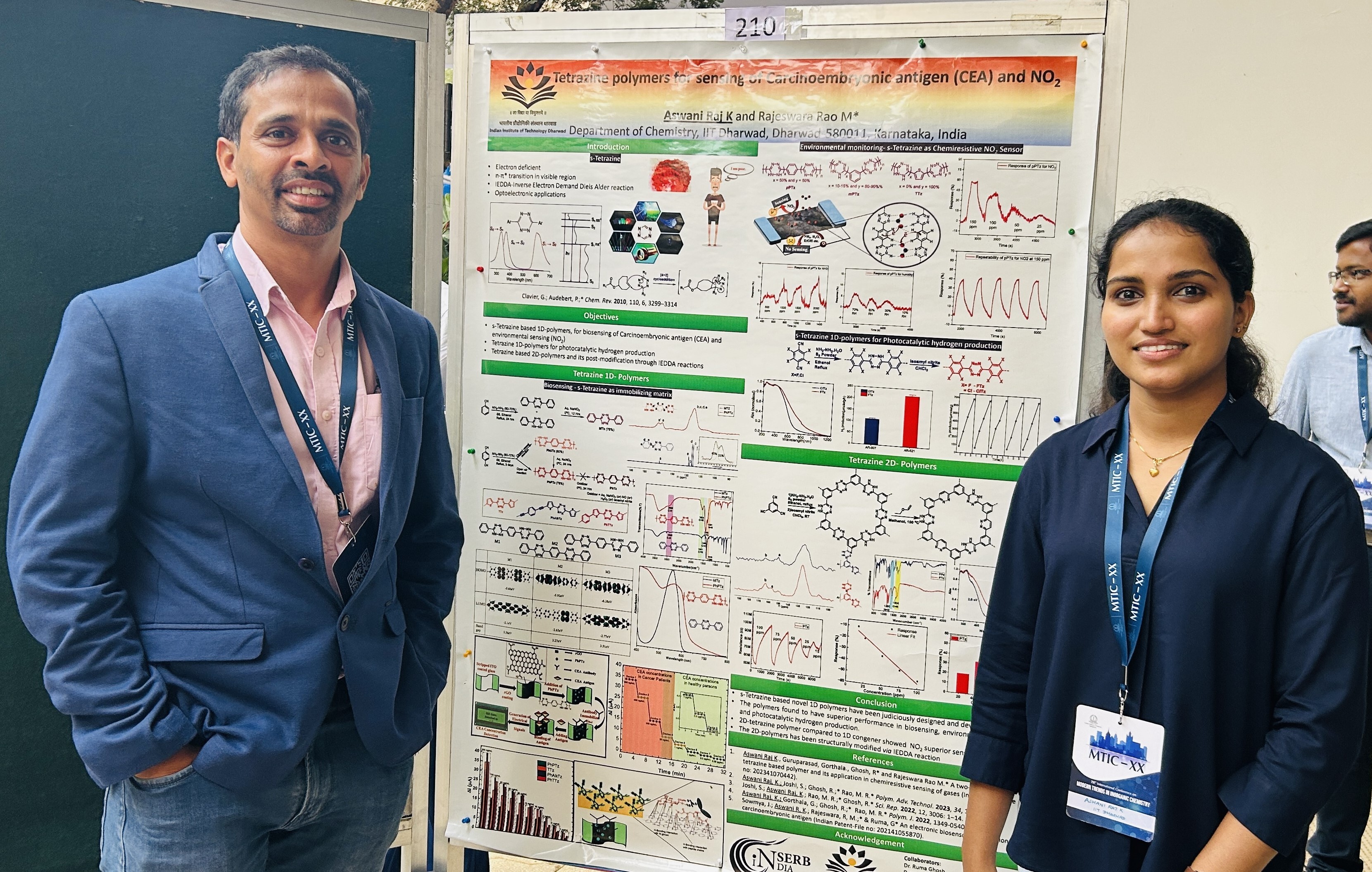
An electronic biosensor based on semiconducting tetrazine polymer immobilizing matrix coated on rGO for carcinoembryonic antigen
DOI:10.1038/s41598-022-06976-0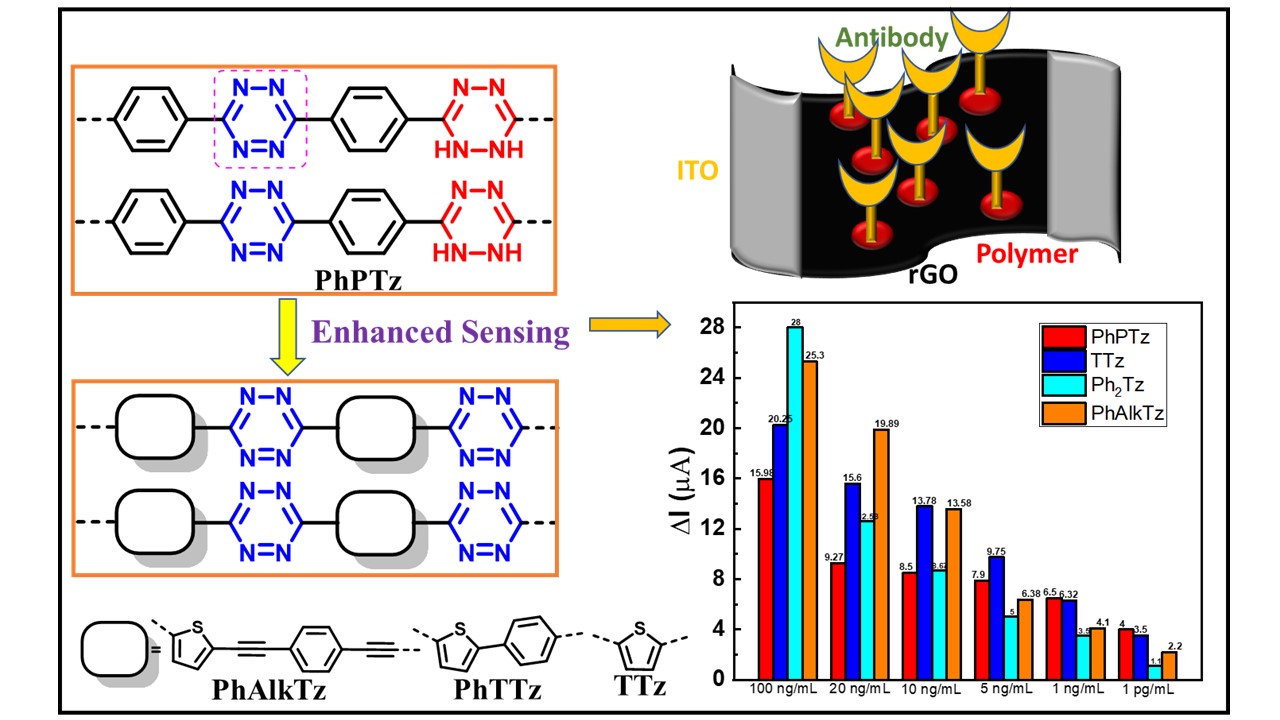
Environmental sensors
An electronic biosensor based on semiconducting tetrazine polymer immobilizing matrix coated on rGO for carcinoembryonic antigen
DOI:10.1038/s41428-022-00667-3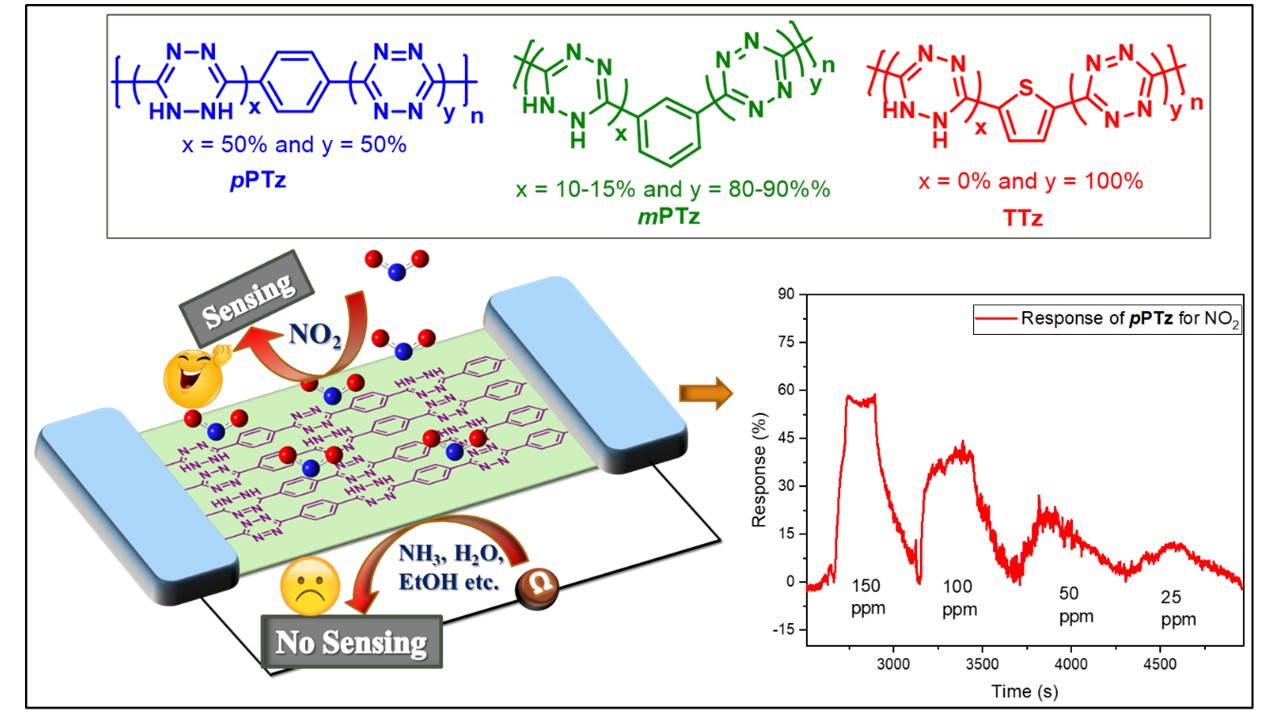
2D π-conjugated molecules
Para-Azaquinodimethane Integrated Quinoidal Conjugated Microporous Polymer
DOI:10.1039/D3TC02233A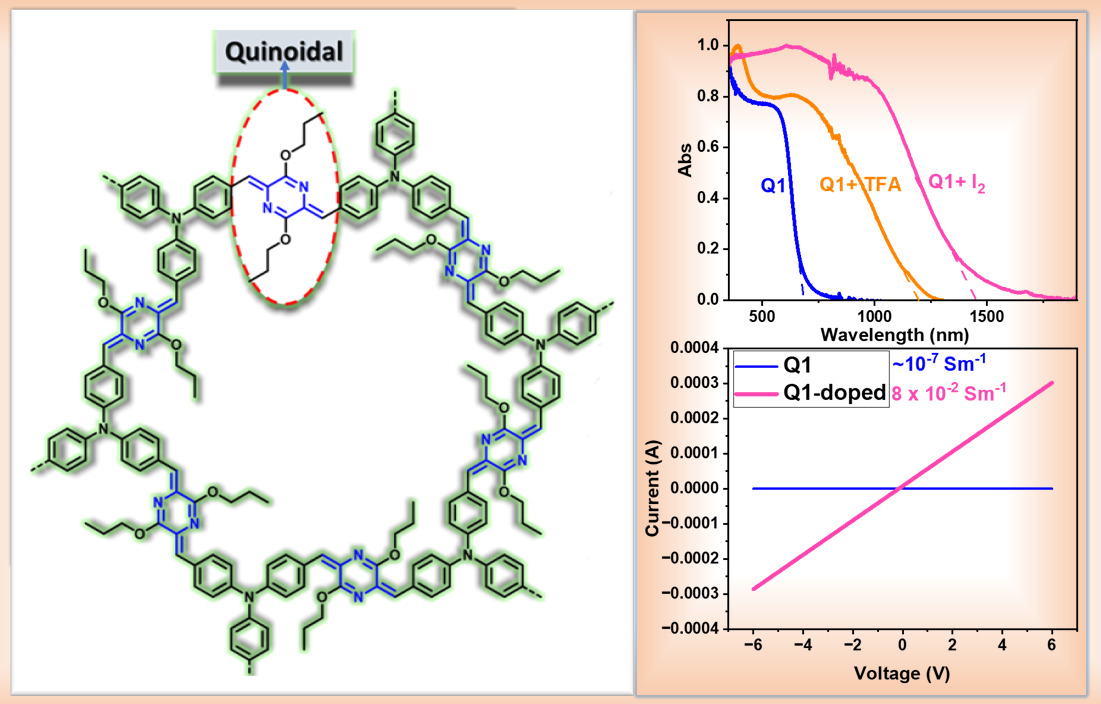
Acid-Modulated Synthesis of Novel π-Conjugated Microporous Polymers for Efficient Metal-Free Photocatalytic Hydrogen Evolution
DOI:10.1038/s41598-022-06976-0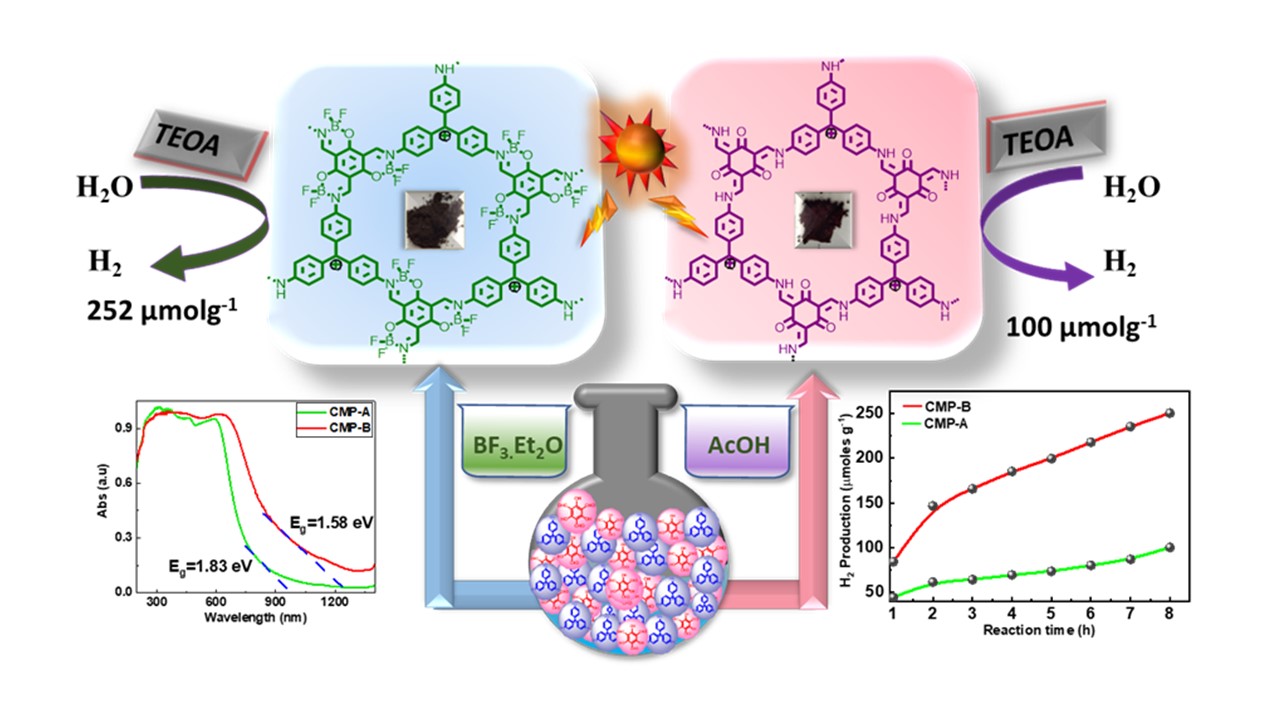
Selectively sensing amines through aldehyde-functional conjugated microporous organic polymers via Pd-catalyzed direct arylation
DOI:10.1038/s41428-022-00736-7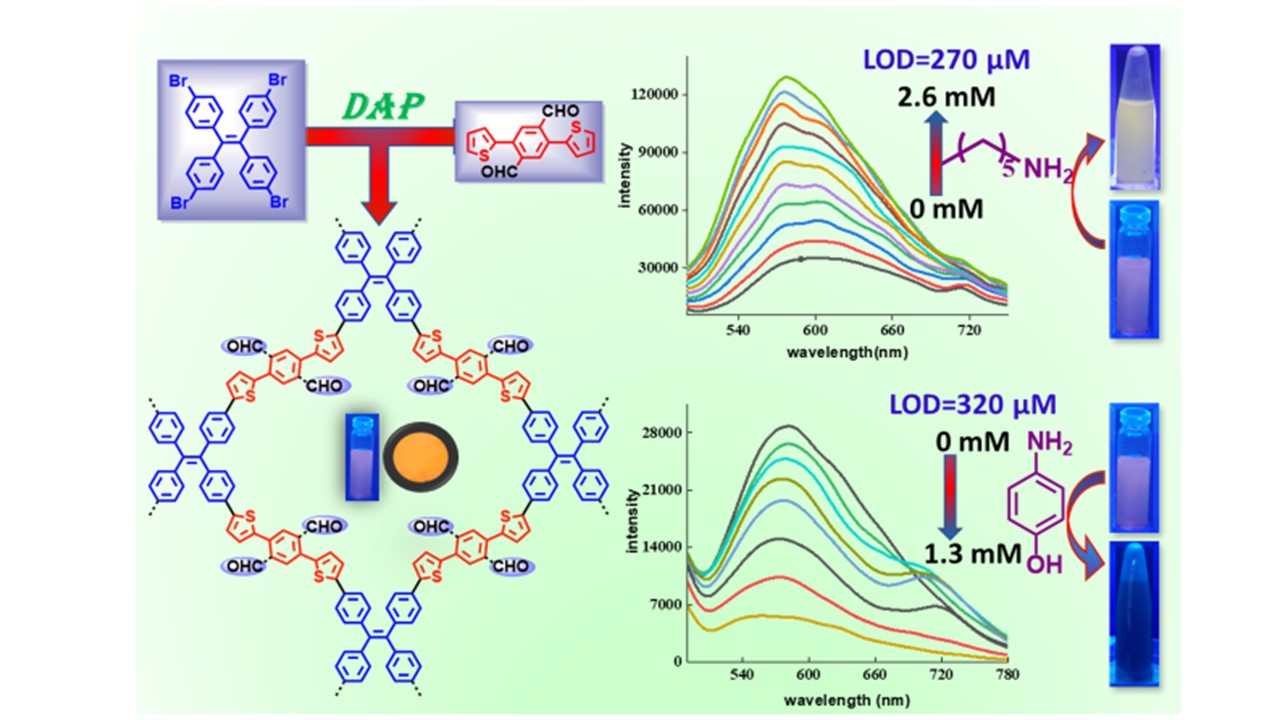
Research Team
Meet the 𝝅-conjugated functional materials laboratory group.

Rajesh
Principle InvestigatorPhD Students

Aswani Raj K
2019 - presentE-mail:193042001@iitdh.ac.in

Atul Babasaheb Nipate
2019 - presentE-mail:193041001@iitdh.ac.in

Vinutha K V
2021-presentE-mail:202053001@iitdh.ac.in

Abhijeet Vitthal Kamble
2021 - presentE-mail:212041001@iitdh.ac.in

Yeshvanth S
2024 - presentE-mail:cy24dp006@iitdh.ac.in

Sharad Pandey
2024 - presentE-mail:cy24dp001@iitdh.ac.in
Project Associate

Chalavadi Chetana
Alumni
Post doctoral fellow
- Aravind R Nesaragi(2023-2024)
- Monika Bai M G(Graduated 2023)
- Apoorva Muragod(June-Oct)
- Potluri Adinarayana(Feb-May, 2023)
- Maharudrayya Pujar (Mar-Sep, 2022)
- BhavaniShankar Gowda Patil(Feb to April, 2022)
- Kashinath Sajjan(Aug-Dec, 2021)
PhD students
Master students
Employement
-
Associate Professor (2023 - Present)
Indian Institute of Technology Dharwad Department of Chemistry -
Assistant Professor (2017 - 2023)
Indian Institute of Technology Dharwad Department of Chemistry -
Post-Doctoral Fellow (2013 - 2016)
McGill University Canada Advisor: Prof. Dmitrii F Perepichka Post-Doctoral Fellow (2011 - 2013)
Academia sinica, Taipei,Taiwan Advisor: Dr. Shih-Sheng Sun
Education
-
PhD (2011)
Department of Chemistry, IIT Bombay Thesis: Synthesis and Studies of Porphyrin and boron-dipyrromethene based fluorescent system Advisor: Prof. M Ravikanth -
M.Sc. (2004)
Andhra University -
B.Sc. (2002)
Andhra University
Publications
Journal Papers
- Aswani Raj K, Subhajit Kar, Santanu Bhattacharyya and Rajeswara Rao Malakalapalli. Effect of Halogenation on Photocatalytic Hydrogen Evolution Performanceof Tetrazine Polymers. ACS Appl. polym. mater. 2024, Accepted. coming soon
- Vinutha K. Venkatareddy and M. Rajeswara Rao* " Vinylene-linked diketopyrrolopyrrole chromophores for electrochromism" RSC Adv., 2024, 14, 10017. https://doi.org/10.1039/D4RA01280A
- Atul B. Nipate and M. Rajeswara Rao*"Pd-Catalysed Direct Arylation of Distyrylbenzene: Strong Dual-state Fluorescence and Electrochromism" Chem. Eur. J. 2024, 30, e202400015. https://doi.org/10.1002/chem.202400015
- K. Aswani Raj and M. Rajeswara Rao*"Para-Azaquinodimethane Integrated Quinoidal Conjugated Microporous Polymer" J. Mater. Chem. C, 2024,12, 110-117.https://doi.org/10.1039/D3TC02233A
- K. Aswani Raj and M. Rajeswara Rao*"Synthesis of p‑Azaquinodimethane-based Quinoidal Fluorophores" J. Org. Chem. 2023, 88, 21, 14960–14968.https://doi.org/10.1021/acs.joc.3c01342
- Bai, M. G. M.; Atul B. Nipate and M. Rajeswara Rao*"Blue-to-Red-Emissive Star-Shaped Boranils" Chemistry select. 2023,8, e202301039. https://doi.org/10.1002/slct.202301039
- Vinutha K. Venkatareddy; Manish Kumar; Vijay Pal Singh and M. Rajeswara Rao* "ESIPT-Active Pyrene-imidazole Fluorophores: Ground-State Intramolecular Proton Transfer (GSIPT), Dual Solid- and Solution-State Emission plus Counter-Intuitive Crystal Packing" ChemPhotoChem. 2023, Advance Article. https://doi.org/10.1002/cptc.202300115
- Abhijeet V. Kamble; K. Aswani Raj and M. Rajeswara Rao* "Synthesis of Tetrazine-Tetracyanobutadienes and Their Transformation to Pyridazines via Inverse-electron Demand Diels-Alder Cycloaddition (IEDDA)" Org. Biomol. Chem. 2023, 21, 5790-5798. DOI https://doi.org/10.1039/D3OB00595J
- Enoch, S., Nipate, A.B. and Vellanki, L.and M. Rajeswara Rao* Croconic acid derived narrow band gap conjugated microporous polymer. Chem Comm. 2023,59, 8846-8849. https://doi.org/10.1039/D3CC01701J
- Atul B. Nipate and M. Rajeswara Rao* "Solid-state red-emissive (cyano)vinylene heteroaromatics via Pd-catalysed C–H homocoupling" Org. Biomol. Chem. 2023, 19, 4123-4129. https://doi.org/10.1039/D3OB00560G
- K. Laxman; Yuxuan Che; K. Aswani Raj; D. F. Perepichka* and M. Rajeswara Rao* "Trifluoroacetic acid promoted unexpected visible to NIR switching of ketoenamine-substituted triphenylamines" J. Mater. Chem. C. 2023, 11, 2680-2687. https://doi.org/10.1039/D2TC04959G
- Bai, M. G. M.; Atul B. Nipate and M. Rajeswara Rao* "Selectively sensing amines through aldehyde-functional conjugated microporous organic polymers via Pd-catalyzed direct arylation" Polym. J. 2023, 55,133–140. https://doi.org/10.1038/s41428-022-00736-7
- K. Aswani Raj;Sowmya Joshi; Ruma Gosh* and M. Rajeswara Rao* "Structural tailoring of semiconducting tetrazine polymers based immobilizing matrix for superior electronic biosensing of carcinoembryionic antigen" Polym. Adv. Technol. 2023, 34, 1331-1340. https://doi.org/10.1002/pat.5973
- Bai, M. G. M.; K. Bramhaiah; S. Bhattacharya * and M. Rajeswara Rao* "Acid-modulated Synthesis of Novel pi-conjugated Microporous Polymers for efficient Metal-free Photocatalytic Hydrogen Evolution" Chem. Eur. J. 2022, e202202023. https://doi.org/10.1002/chem.202202023
- K. Aswani Raj; Gorthala Guruprasad; Ruma Gosh* and M. Rajeswara Rao* "Tetrazine-based 1D polymers for the selective chemiresistive sensing of nitrogen dioxide via the interplay between hydrogen bonding and n-heteroatom interactions" Polym. J. 2022, 54, 1191-1201. https://doi.org/10.1038/s41428-022-00667-3
- Sowmya Joshi; K. Aswani Raj; M. Rajeswara Rao* and Ruma Gosh* "An electronic biosensor based on semicondcuting tetrazine polymer immobilizing matrix coated on rGO for carcinaembryonic antigen" Sci. Rep. 2022, 12, 1-14. doi: 10.1038/s41598-022-06976-0
- Bai, M. G. M.; Babu, H. V.; V. Lakshmi* and M. Rajeswara Rao* "Structure–property–function relationship of fluorescent conjugated microporous polymers" Mater. Chem. Front. 2021, 5, 2506−2551. https://doi.org/10.1039/D0QM00769B
- G. Galeotti, F. De Marchi, E. Hamzehpoor, O. MacLean, M. Rajeswara Rao, Y. Chen, L. V. Besteiro, D. Dettmann, L. Ferrari, F. Frezza, P. M. Sheverdyaeva,R. Liu, A. K. Kundu, P. Moras, M. Ebrahimi, M. C. Gallagher, F. Rosei, D. F. Perepichka, G. Contini. "Synthesis of mesoscale ordered 2D π-conjugated polymer with semiconducting properties" Nature. Mater. 2020, 19, 874. https://doi.org/10.1038/s41563-020-0682-z
- Lakshmi, V.; Liu, C.-H.; Rajeswara Rao, M.; Chen, Y.; Yuan, F.; Hamzehpoor, E.; Sakai-Otsuka, Y.; Stein, R. S.; Perepichka, D. F. "A two-dimensional poly (azatriangulene) covalent organic framework with semiconducting and paramagnetic state" J. Am. Chem. Soc. 2020, 142, 2155. https://doi.org/10.1021/jacs.9b11528
- De Marchi, F.; Galeotti, G.; Simenas, M.; Gallagher, M.; Hamzehpoor, E.; MacLean, O.; Rajeswara Rao, M.; Chen, Y.; Dettmann, D.; Contini, G.; Tornau, E.; Ebrahimi, M.; Perepichka, D. F.; Rosei, F. "Temperature induced Molecular reorganization on Au(111) driven by oligomeric Defects" Nanoscale. 2019, 11, 19468. https://doi.org/10.1039/C9NR06117G
- Babu, H. V.; Bai, M. G. M. and Rajeswara Rao, M.* "Functional π-Conjugated Covalent Organic Frameworks" ACS Appl. Mater. Interfaces 2019, 11, 11029. https://doi.org/10.1021/acsami.8b19087
- Isar, P.; Rajeswara Rao, M.; Ravikanth, M. “Synthesis, Characterization, Sensing and Coordination Properties of Trans-Homoporphodimethenes” Eur. J. Org. Chem. 2018,. https://doi.org/10.1002/ejoc.201800264
- Kumar. S.; Rajeswara Rao, M.; Ravikanth, M. “Stable Core-modified Doubly N-confused Expanded Dibenzoporphyrinoids” J. Org. Chem. 2018, 83, 1584. https://doi.org/10.1021/acs.joc.7b02851
- Alka, A.; Pareek, Y.; Shetti, V. S.; Rajeswara Rao, M.; Theophall, G. G.; Lee, W. Z.; Lakshmi, K. V.; Ravikanth, M. “Construction of Novel Cyclic Tetrads by Axial Coordination of Thiaporphyrins to Tin(IV)Porphyrins” Inorg. Chem. 2017, 56, 13913. https://doi.org/10.1021/acs.inorgchem.7b01966
- Kumar, A.; Rajeswara Rao, M.; Lee, W. Z.; Ravikanth, M. “Hybrid Macrocycles of Subporphyrins and Triphyrins” Org. Lett. 2017, 19, 5924. https://doi.org/10.1021/acs.orglett.7b02919
- Sharma, R.; Rajeswara Rao, M.; and Ravikanth, M. “a-Pyrrolyl Dipyrrins as Suitable Ligands for Coordination chemistry” Coord. Chem. Rev. 2017, 348, 92. https://doi.org/10.1016/j.ccr.2017.08.002
- Lijia. L; Filip. P; Rajeswara Rao, M; and Perepichka, D. F.* “A Wide Bandgap Naphthalene Semiconductor for Thin-film Transistors” Adv. Electron. Mater. 2017, 3, 1600556. https://doi.org/10.1002/aelm.201600556
- Rajeswara Rao, M; Fang, Y.; DeFeyter, S. and Perepichka, D. F.* “Conjugated Covalent Organic frameworks via Michael Addition-Elimination” J. Am. Chem. Soc. 2017, 139, 2421. https://doi.org/10.1021/jacs.6b12005
- Rajeswara Rao, M; Johnson, S.; and Perepichka, D. F.* “Aromatization of Benzannulated Perylene-8,16-diones: Photophysical Properties and Reactivity” Org. Lett. 2016, 18, 3574-3577. https://doi.org/10.1021/acs.orglett.6b01559
- Rajeswara Rao, M; Black, H. T.; and Perepichka, D. F.* “Synthesis and divergent electronic properties of two ring-fused derivatives of 9,10-diphenylanthracene” Org. Lett. 2015, 17, 4224-4227. https://doi.org/10.1021/acs.orglett.5b02009
- Rajeswara Rao, M; Desmecht, A and Perepichka, D. F.* “π-Extended indenofluorenes” Chem. Eur. J. 2015, 21, 6193-6201. https://doi.org/10.1002/chem.201406646
- Chia-Wei Liao; Rajeswara Rao, M and Shih-Sheng Sun,* “Structural diversity of new solid-state luminophores based on quinoxaline--ketoiminate boron difluoride complexes with remarkable switching properties” Chem. Commun. 2015, 51, 2656-2659. https://doi.org/10.1039/C4CC08958H
- Lakshmi, V; Rajeswara Rao, M and Ravikanth, M,* “Halogenated Boron-dipyrromethenes: Synthesis, Properties and Applications” Org. Biomol. Chem. 2015, 13, 2501-2517. https://doi.org/10.1039/C4OB02293A
- Kaur, T; Rajeswara Rao, M and Ravikanth, M,* “Multi-porphyrin arrays on cyclotriphosphazene scaffolds” Inorg. Chem. 2014, 53, 11051-11059. https://doi.org/10.1021/ic501569e
- Rajeswara Rao, M and Shih-Sheng Sun,* “Supramolecular assemblies amide-derived organogels featuring rigid π-conjugated phenylethynyl frameworks” Langmuir 2013, 29, 15146-15158 (Invited Feature article). https://doi.org/10.1021/la402449e
- Rajeswara Rao, M.; Chia-Wei Liao and Shih-Sheng Sun,* “Structurally simple thienodipyrandione-containing reversible fluorescent switching piezo- and acido-chromic materials” J. Mater. Chem. C 2013, 1, 6386-6394. DOI https://doi.org/10.1039/C3TC31504E
- Rajeswara Rao, M.; Chia-Wei Liao.; Wei-Lin Su and Shih-Sheng Sun,* “Quinoxaline based D-A-D molecules: high contrast reversible solid-state mechano- and thermo- responsive fluorescent materials” J. Mater. Chem. C 2013, 1, 5491-5501. DOI https://doi.org/10.1039/C3TC31179A
- Rajeswara Rao, M and Ravikanth, M,* “Synthesis of functionalized core-modified sapphyrins and covalently linked porphyrin-sapphyrin dyads” Tetrahedron 2012, 68, 1306-1314. https://doi.org/10.1016/j.tet.2011.11.030
- Khan, T. K.; Jana, S. K.; Rajeswara Rao, M.; Shaikh, M. S. and Ravikanth, M.* “Synthesis and electronic properties of meso-furyl boron-dipyrromethenes” Inorg. Chim. Acta 2012, 383, 257-266. https://doi.org/10.1016/j.ica.2011.11.017
- Rajeswara Rao, M.; Manu T. T, Suresh, B. and Ravikanth, M.* “Synthesis of BF2 complexes of prodigiosin type oligopyrroles” J. Org. Chem. 2011, 76, 7263-7268. https://doi.org/10.1021/jo201183s
- Rajeswara Rao, M. and Ravikanth, M.* “Boron complexes of oxasmaragdyrin, a core-modified expanded porphyrin” J. Org. Chem. 2011, 76, 3582-3587. https://doi.org/10.1021/jo200295b
- Madhu, S.; Rajeswara Rao, M.; Shaikh, M. S. and Ravikanth, M.* “3, 5-Diformyl Boron-dipyrromethenes as Fluorescent pH sensors” Inorg. Chem. 2011, 50, 4392-4400. https://doi.org/10.1021/ic102499h
- Rajeswara Rao, M.; Ghosh, A. and Ravikanth, M.* “Synthesis, spectral and electrochemical properties of cyclotriphosphazene appended with six metalloporphyrins” Inorg. Chim. Acta 2011, 372, 436-441.https://doi.org/10.1016/j.ica.2011.03.021
- Rajeswara Rao, M. and Ravikanth, M.* “Synthesis and anion binding studies of covalently linked porphyrin-expanded heteroporphyrin dyads” Eur. J. Org. Chem. 2011, 1335-1345. https://doi.org/10.1002/ejoc.201001482
- Khaderbad, M. A.; Roy, U.; Yedukondalu, M.; Rajeswara Rao, M.; Ravikanth, M.* and Rao, V. R. “Variable interface dipoles of metallated porphyrin self-assembled monolayers for metal-gate work function tuning in advanced CMOS technologies” IEEE Transactions on Nanotechnology 2010, 9, 335-337. 10.1109/TNANO.2010.2043681
- Khan, T. K.; Rajeswara Rao, M. and Ravikanth, M.* “Synthesis and photophysical properties of 3,5-bis(oxopyridinyl)- and 3,5-bis(pyridinyloxy)-substituted boron-dipyrromethenes” Eur. J. Org. Chem. 2010, 2314-2323. https://doi.org/10.1002/ejoc.200901460
- Rajeswara Rao, M.; Pavan Kumar, K. V. and Ravikanth, M.* “Synthesis of boron-dipyrromethene ferrocene conjugates” J. Organomet. Chem. 2010, 695, 863-869. https://doi.org/10.1016/j.jorganchem.2010.01.009
- Rajeswara Rao, M.; Mobin, S. M. and Ravikanth, M.* “Boron-dipyrromethene based specific chemodosimeter for fluoride ion” Tetrahedron 2010, 66, 1728-1734. https://doi.org/10.1016/j.tet.2009.12.039
- Rajeswara Rao, M.; Bolligarla, R.; Butcher, R. J. and Ravikanth, M.* “Hexa boron-dipyrromethene cyclotriphosphazenes: Synthesis, crystal structure, and photophysical properties” Inorg. Chem. 2010, 49, 10606-10616.https://doi.org/10.1021/ic1016092
- Rajeswara Rao, M.; Gayatri, G.; Amit, K.; Sastry, G. N.* and Ravikanth, M.* “Cyclotriphosphazene ring as a platform for multiporphyrin assemblies” Chem. Eur. J. 2009, 15, 3488-3496. https://doi.org/10.1002/chem.200802413
Patents
- Vinutha K.V, Spoorti M, Ruma Gosh*,and M Rajeswara Rao,* “Chemiresistive sensor based on two dimensional dicationic Bipyridyl Vinylene-linked polymer” Indian patent, 2024 (Application No. 202441024492).
- Spoorti M, Vinutha K V, M Rajeswara Rao,*and Ruma Gosh,* “Resistive Sensor based on one dimensional pi-conjugated dicationic 4,4’- bipyridine polymer and method” Indian patent, 2024(Application No.202441024489).
- K Aswani Raj, Guruprasad Gorthala, Ruma Gosh*,and M Rajeswara Rao,* “A two-dimensional tetrazine based polymer and its application in chemiresistive sensing of gases” Indian patent, 2023 (File no: ).
- Sowmya Joshi, K Aswani Raj,and M Rajeswara Rao,* and Ruma Gosh* “An electronic biosensor for detection of carcinoembryonic antigen” Indian patent, 2021 (File no: ).
- Rajeswara Rao, M. and Ravikanth, M.* “A simple method for the synthesis of biocompatible 3-pyrrolyl boron-dipyrromethenes” Indian patent, 2011 (File no: 1443/mum/2011).
Book Chapters
- Aswani Raj K, Rajeswara Rao, M. * Book chapter: Crystalline Two-dimensional Organic Porous Polymers (Covalent Organic Frameworks) for Photocatalysis. Title of the book: Materials Science in Photocatalysis, Elsevier 2021.
- Rajeswara Rao, M. and Shih-Sheng Sun.* “Supramolecular assemblies of organogels featuring pi-conjugated framework with long-chain dicarboxamides” Chapter, Pan Stanford Publishing, 2012.
Teaching
UG Courses
- Introduction to sophisticated charcterization technique
- Chemistry for engineers (CH101)
- Chemistry laboratory (CH 111)
- Sustainable energy and energy materials
PG Courses
- Topics in chemistry
- Optical and electronic properties of π-conjugated compounds
- Organometallic chemistry
- Battery technology and thermal management
Photo Gallery





















News
- Hearty congratulations Atul for paper.
- Hearty congratulations Aswani for JMCC paper.
- Hearty congratulations Aswani for the patent.
Contact
You may contact me through email or over phone. To join our research group, send an email to me and keep watching at the institute webpage for vacancies.
Location:
Department of Chemistry, IIT Dharwad, WALMI campus, Karnataka, India 580011.
Email:
rajesh@iitdh.ac.in
Call:
+91 836 2212 834
Designed by BootstrapMade.
