Research interests
Our research interests lie in the field of bioorganic chemistry and chemical biology. We study chemistry of enzyme catalysed reactions in the natural product biosynthetic pathways, which include ribosomal as well as non-ribosomal peptides. We employ interdisciplinary techniques from chemistry and biology and use various spectroscopic methods to gain insights into the molecular details of the reactions catalysed by enzymes. We are currently working on following research areas.
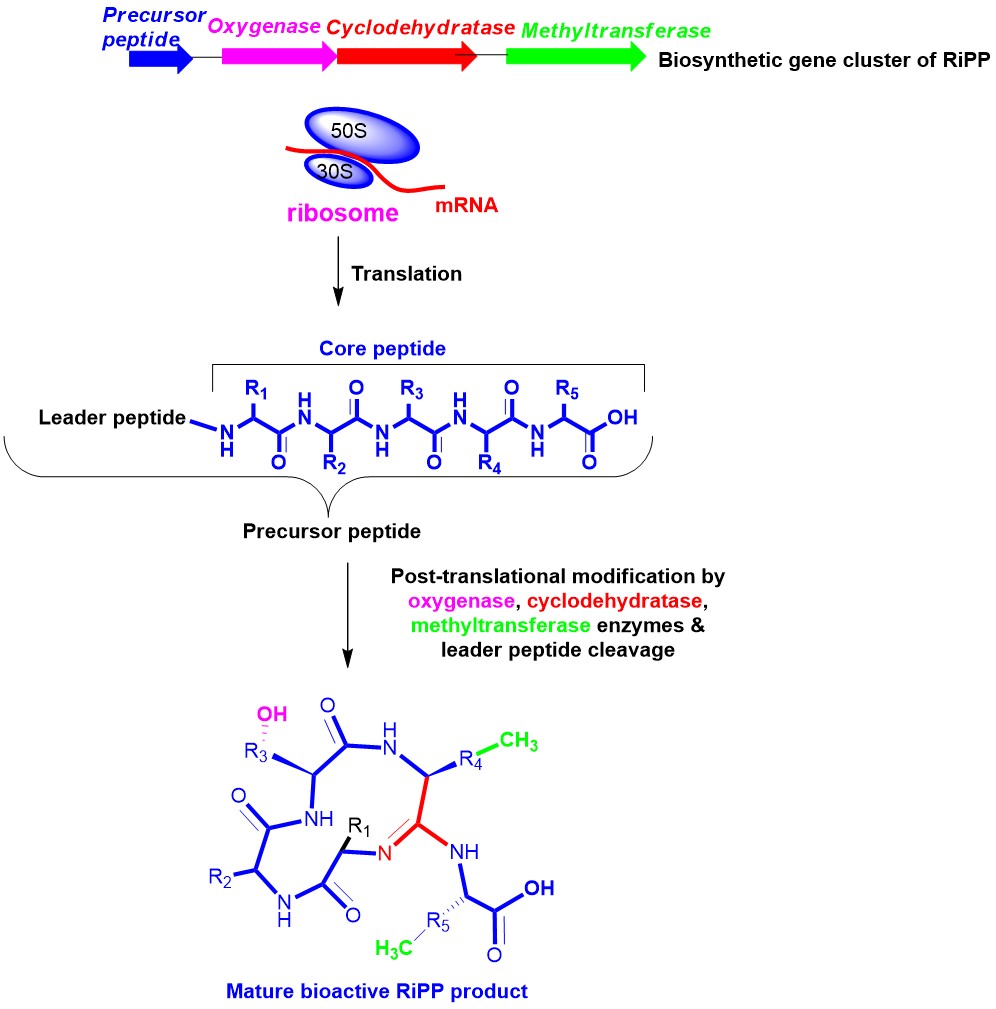
Organic chemistry of the enzyme catalysed reactions in RiPP biosynthesis:
In today’s world, the emergence of antibiotic resistance in bacteria is proving to be a serious and increasing threat to human health. Therefore, discovery of new structural motifs with novel antibacterial targets exhibiting activity against multi-drug resistant pathogens is of utmost importance. Ribosomally synthesized and post-translationally modified peptide natural product (RiPP) produced by bacteria, are an emerging class of peptide derived compounds with diverse structural features exhibiting wide array of bioactivities ranging from antibacterial to anticancer properties. RiPP precursor peptides are direct ribosomal gene products that undergo various post-translational modifications (PTMs) by enzymes to synthesize the mature, structurally complex antibiotics with novel mechanisms of action. RiPPs offer high degree of malleability for bioengineering to produce a library of antibiotic analogs.
Studying the biosynthetic pathway offers an opportunity to investigate the chemistry of various enzymes. These include C-H activation by radical S-adenosyl methionine (rSAM) enzymes, non-heme iron dependent rieske oxygenase and cytochrome P450, macrocyclization/heterocyclization/thioamidation by adenosine triphosphate dependent YcaO enzymes, and oxidative decarboxylation by cytochrome P450 enzyme etc. We employ multidisciplinary techniques from chemistry and biology to gain insights into the molecular details of these mechanisms. Studying the enzymatic mechanism will help us to engineer biosynthetic pathways for generating antibiotic analogs in the long term. We are currently working on a RiPP antibiotic bottromycin which has unique mechanism of action targeting protein synthesis.
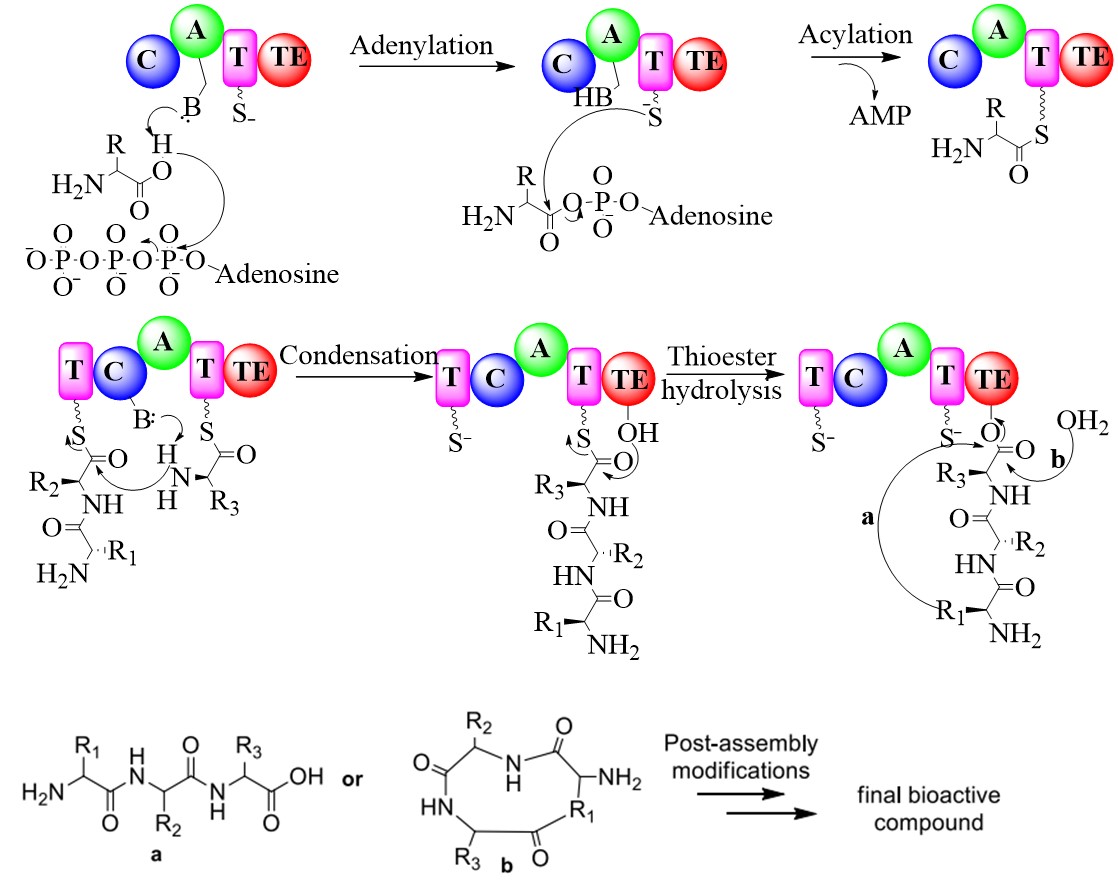
Enzymology of bioactive NRP/PK natural products:
Nonribosomal peptide (NRPs) natural products are short peptides produced by large multimodular enzymes. They are a kind of peptide secondary metabolites which are synthesized by multidomain mega-enzymes named nonribosmal peptide synthetases (NRPSs), without the need for the cell ribosomal machinery and messenger RNAs. NRPs are naturally synthesized by microorganisms such as bacteria and fungi, but also by the symbionts of higher eukaryotes. NRPs represent a vast range of bioactivities and pharmacological properties. For example, NRP natural products such as antibiotics (e.g., actinomycin, penicillin, cephalosporin, vancomycin), cytotoxics (e.g., bleomycin), and immunosuppressants (e.g., cyclosporines) have found beneficial applications in human medicine. Multi-domain NRPS contain adenylation (A) domain, which activate amino/aryl acids to amino/aryl acyl-AMP. NRPS elongations involve C-N bond formation as an amide (peptide) link is forged in each condensation step by condensation domain. The thioesterase (TE) domain hydrolyse completed chain from thiolation (T) domain of the previous module in termination step followed by the post-assembly modification to bioactive non-ribosomal peptide.
Polyketides (PKs) are a large class of structurally diverse, acetate derived natural products that exhibit a wide range of bioactivities. They are biosynthesized by a complex collection of enzymes known as a polyketide synthases (PKSs). The process takes place by iterative thio-Claisen condensations of malonyl-CoA and/or its derivatives in a manner analogous to fatty acid biosynthesis to produce β-keto thioester intermediates. We are currently working on a interesting hybrid NRP-PK derived broad spectrum antibiotic which exhibit activity against both gram positive and gram negative pathogens by targeting cell membrane and central metabolic pathways.
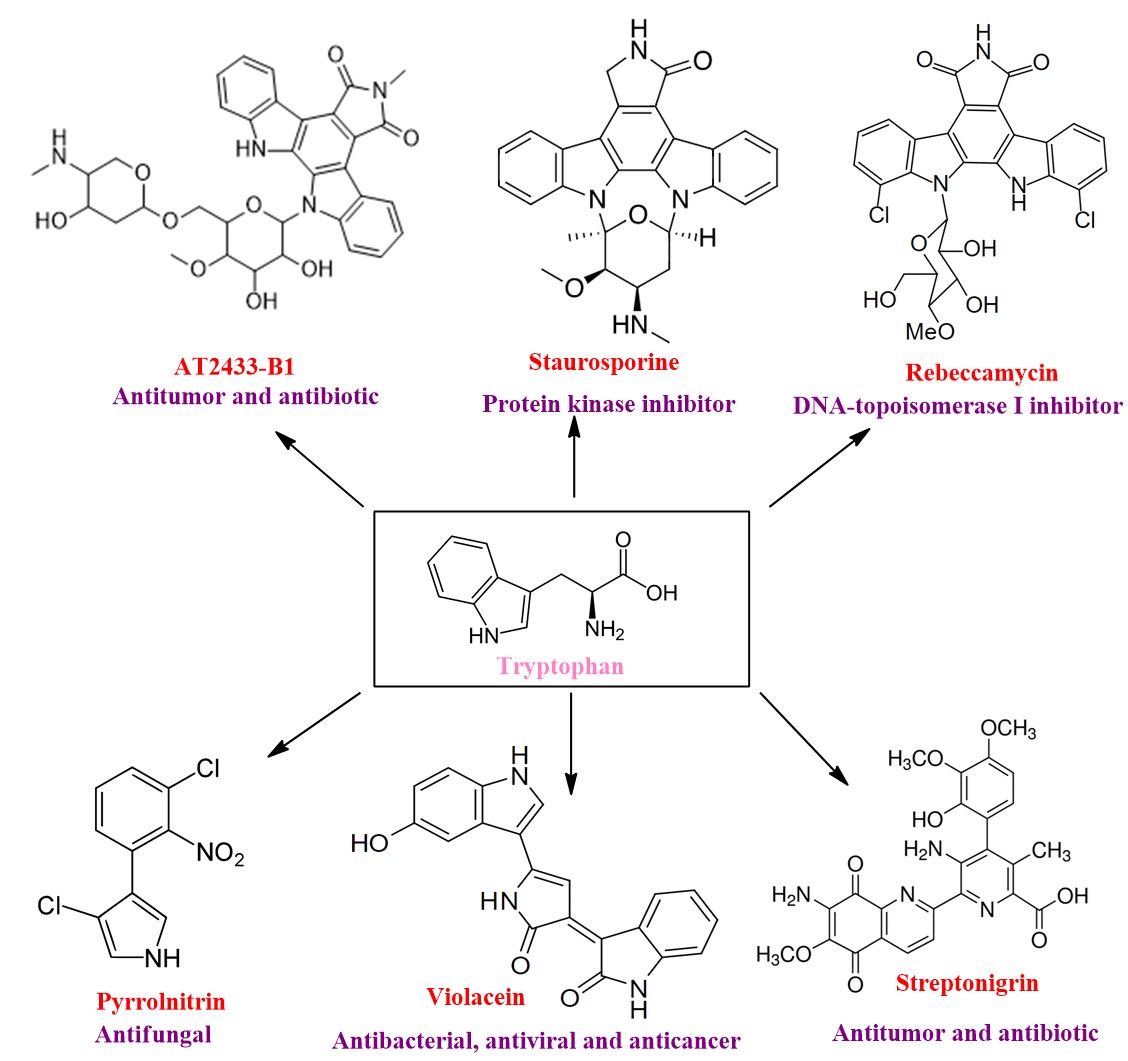
Investigation of the biosynthesis of tryptophan derived bioactive natural products:
Tryptophan, the most chemically complex and the least abundant of the 20 common proteinogenic amino acid. Tryptophan is a biosynthetic precursor to many complex microbial natural products (such as antibiotic pyrrolnitirin), which are promising scaffolds for drug discovery and development.The chemical features of tryptophan, including its ability to undergo chemistry at almost every atom makes it a unique biological precursor for the generation of chemical complexity. Recently it was discovered that tryptophan leads to the formation of a few novel anticancer compounds of highly functionalized alkaloid family which were shown to induce DNA single and double strand breaks and metal dependent DNA complex formation. Moreover, they also cause several chromosomal aberrations, and blocks the synthesis of DNA and RNA by inhibiting topoisomerase II.Our goal would be to study the chemistry of the biosynthetic machineries (synthesis by enzymes in a biological set up) of such natural products in-vitro, understand the fundamental principles of the complex organic/inorganic chemical reactions (they also involve metalloenzymes) and characterize the molecular details of these proteins using various techniques from chemistry (synthetic chemistry, bioorganic/bioinorganic and biophysical chemistry) and biology (protein biochemistry, bioinformatics, molecular and cell biology).
Other than these areas, we are also interested in studying biofuels (mainly microalgae and plant derived) for clean bioenergy applications in collaboration with colleagues from mechanical and electrical engineering departments of IIT Dharwad.